EnviroMail 93 Australia
Laboratory quality assurance/quality control assessment to assist project data quality review and reporting
Quality indicators are central to evaluating the efficacy of any data-based report, and yet may seem cryptic to the unfamiliar eye. This EnviroMail is aimed to assist in the understanding and interpretation of data quality parameters and what their significance might hold regarding data validity and ultimately decision making in the contexts that associated data is applied.

Quality assurance
Quality assurance (QA) involves planned and systematic actions, procedures, checks, and decisions undertaken to ensure the representativeness and integrity of samples collected for analysis, and the accuracy and reliability of the analytical results.
In the field QA measures include:
- selection of appropriate sampling and preservation methods, sample containers and sample storage
- decontamination procedures such as cleaning of tools before sampling and between samples
- maintenance of the sample environment to minimise sample contamination and analyte losses
- delivery to the laboratory in good condition and within the timeframes required for the analytes
Quality control
Quality control (QC), encompasses all the systems of control in the field and laboratory for the monitoring the efficacy of the quality assurance procedures. (NEPM 2013).
Common quality control at ALS laboratories
Quality control is a crucial component in providing reliable and defensible data. Quality control is implemented in our laboratories by utilising appropriate, industry standard QC sample types, which demonstrate control of laboratory processes and confirm that tests are performing as anticipated. QC samples are introduced at critical points of sample handling, preparation, and analysis.
Instrument QC (e.g. calibration checks) demonstrates control of the instrumental portion of a method. Instrument QC requirements must be met before the analysis of client samples and associated method-based QC.
Method QC encompasses the entire test method, including all sample preparation and instrumental analysis factors.
Appropriate method QC depends on the type of test, but often includes method blanks (MB), duplicates (DUP), laboratory control samples (LCS), Matrix Spikes (MS), Surrogates, and reference materials (RM). The evaluation of Method QC sample results is important to verify the quality of associated sample test results.
| QC type | Definition and purpose |
| Method blank | A blank matrix that has gone through the same analysis steps as samples, used to monitor variability of the blank response, contamination, and bias. |
| Duplicate | A second aliquot of sample analysed independently from the primary test sample. Assesses variance (precision) of the total method including lab sub-sampling and analysis. |
| Laboratory control samples | A clean matrix that has been fortified with a known amount of target analyte, used to verify the accuracy (precision and bias) of the test method. |
| Matrix spike | An aliquot of client sample fortified with a known amount of target analyte, used to identify matrix interference effects on method recovery. |
| Surrogate | An organic compound with similar chemical composition and properties to the target analyte(s), used to monitor analyte recovery / extraction efficiency in each sample. Commonly used in organics testing. |
| Reference material | A material or substance with homogenous and well-established properties, used to assess the performance of a measurement method. |
Data quality objectives
ALS Data Quality Objectives (DQOs) are performance specifications that are defined by QC control limits. DQOs are established for each QC type, based on a combination of reference method objectives, customer requirements, and historical test method performance.
Holding times and causes of analyte change
Another important aspect of data quality evaluation is consideration of holding times. Holding times are the maximum recommended times elapsed between sample collection and commencement of preparation or analysis. Holding times are based on published reference methods, industry standards, and jurisdictional requirements. Meeting holding times is important to ensure that measured analyte concentrations reflect the condition of the sample at time of collection as best as possible. However, it should be noted that holding times prescribed by many sources can seem arbitrary and typically represent a worst-case scenario, without consideration of the nature of the samples in question.
This problem has been recognised by the US EPA and was the impetus for a 2006 EPA study titled “Sample Holding Time Re-Evaluation”. The EPA introduction to this project stated the following:
“While holding times may appear adequate to protect sample integrity and provide sufficient time for laboratory analysis, relevant data is sparse on individually defined holding times and, thus, some of the holding times appear to be arbitrary and/or politically driven. Holding times appear to be arbitrary when a single value is applied over a large general class of compounds (e.g., pesticides or polyaromatic hydrocarbons); when the holding time was originally "established" for aqueous media and then blindly applied to other media (e.g., sediments and tissues); or when a contaminant is known to be chemically highly stable and will still be present in the sample even if the sample is not extracted in the regulatory time frame. For example, if PCBs significantly degraded after seven days, then there would not be an environmental problem with PCBs today.”
There are many reasons why parameter concentrations within environmental samples can change over time after sampling, even where appropriate preservation techniques can be applied. Some common reasons are listed below, including whether a negative or positive bias may occur:
- Elevated temperature (false negative)
- Headspace for VOCs (false negative)
- Bacterial degradation (e.g. TRH or nutrients such as AmmoniaNitriteNitrate; negative or positive bias)
- Oxidation or photodegradation (negative or positive bias)
- Metals precipitation or adsorption to bottles (negative bias)
- Microbial morbidity or continued growth (false negative or positive)
- Incorrect field techniques, often involving filtration of metals (false positive or negative).
- Decanting waters (sediment exclusion) and not solvent rinsing bottles for SVOCs, e.g., PAHs (false negative)
While exceeding holding times does not necessarily mean results are no longer valid, additional context and information about samples in questions are required to facilitate inferences on how exceedances may affect the sample results.
How ALS assists in project data quality review
ALS provides important information to clients in the form of several reports designed to confirm whether project Data Quality Objectives have been achieved. Given the risk of negative and positive bias, it is crucial that samples are received with appropriate sample containers, preservation, temperature, and within applicable holding times. ALS provides an early assessment of these factors at the time of sample receipt, which are reported via our sample receipt notification (SRN) as samples are being logged into the laboratory information management system (LIMS).
ALS also offers several other reports that provide information summarising data associated with the entire testing process.
ALS quality control interpretive report
The ALS QCI report automatically generates an at-a-glance summary evaluation and interpretation of QC results and other critical quality elements associated with the testing process. This report highlights exceptions and outliers to ALS data quality objectives, holding time compliance, QC sample frequencies, and lists important methodology references and summaries.
Data quality objective compliance
It is ALS’s objective that all QC results should fall within established control limits. If exceedances occur, appropriate action is taken, including re-extraction and/or reanalysis. Sometimes this not possible due to sample volume constraints, in which case the associated test results are qualified and a comment added to the certificate of analysis. The Summary of Outliers section of the QCI report (Figure 1) indicates whether any DQO exceedances have occurred; if outliers exist, details are provided.
Summary of outliers
Outliers: Quality control samples
- No method blank value outliers occur
- No duplicate outliers occur
- No laboratory control sample (LCS)outliers occur
- No test sample surrogate recovery outliers exist
Outliers: Reference material (RM) samples
- No reference material (RM) sample outliers occur
Outliers: Analysis holding time compliance (breaches)
- No analysis holding time outliers exist
Outliers: Frequency of quality control samples
- No quality control sample frequency outliers occur
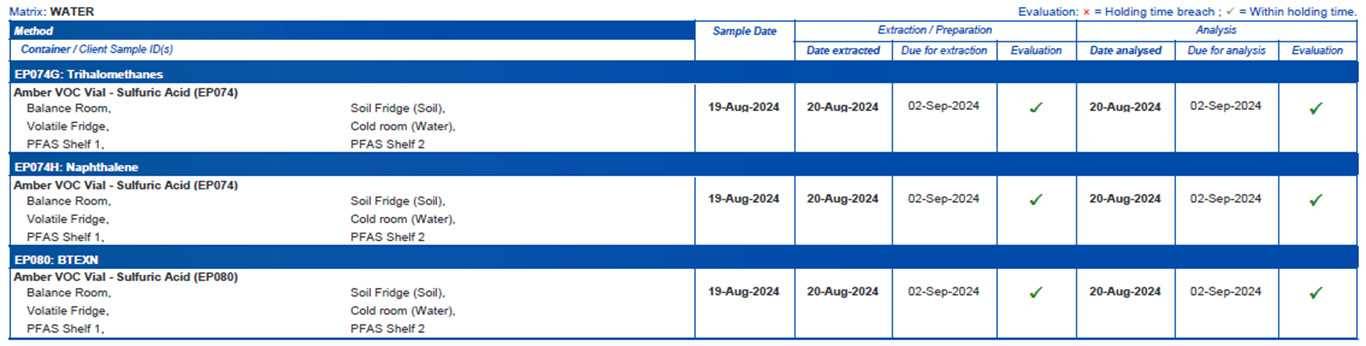
Analysis holding time compliance
The holding time compliance section of the QCI report summarises compliance and exceedances, and includes sample dates, and laboratory preparation and/or analysis dates.
QC sample frequency compliance
QC samples are analysed in analytical batches (QC lots) of client samples. The expected frequency of QC analysis is defined by each test method for each QC type. This section of the QCI report (Figure 3) indicates whether the expected frequency of each QC type was met. Where the actual frequency of QC is greater than or equal to the expected frequency, the evaluation will display a pass. If the frequency is lower, the evaluation will show it to be outside of the specification. Some tests require additional sample volume and containers to facilitate analysis of quality control samples at the recommended frequencies.
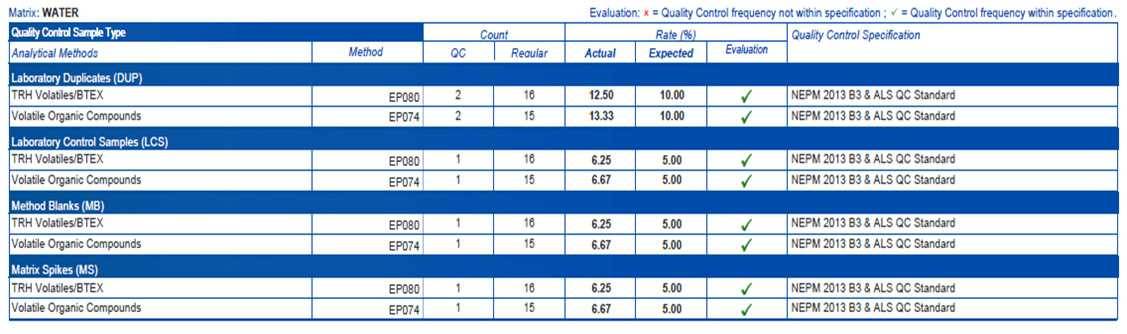
Figure 3: QC Frequency compliance evaluation from QCI Report
Methodology references and summaries
Analytical methods used by ALS are developed using nationally or internationally recognised reference methods, where available. The methodology section of the QCI report (Figure 4) outlines which analytical methods were used for analysis, including a general description of the test. Modifications are fully validated and are intended to provide superior data quality.
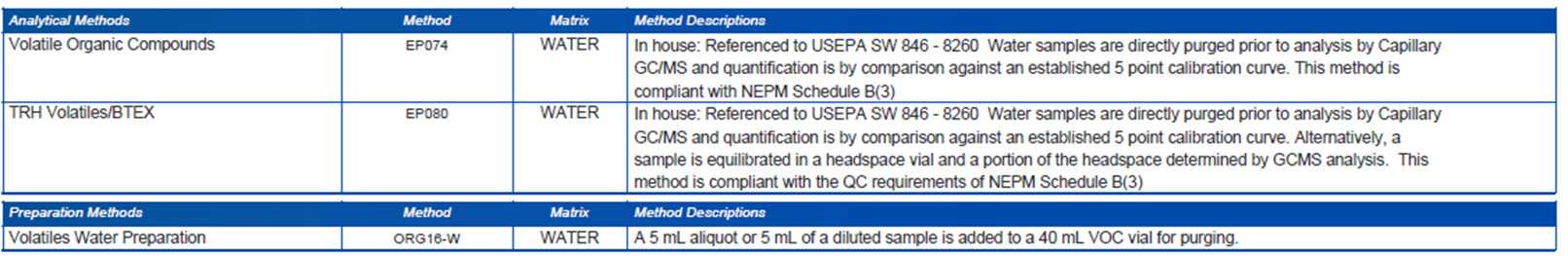
Figure 4: Methodology Summary from QCI Report
Other QC report options
ALS offers a wide array of reports designed to meet your project needs. The QCI report is just one such option and is intended to make review of Holding Time and QC DQO compliance easy. Additional QC report types are also available, including a comprehensive QC Report and Excel Report. Both reports include all QC results associated with sample testing, where more complete information is needed.
Get in touch with us
Contact your local ALS client services to ensure your projects are configured to provide the QC reports that best meet your needs.
Contact us at one of our leading environmental services laboratories on the details below.
Brisbane
Sydney
Melbourne
Perth
Download as a PDF