EnviroMail 158 Australia
Lifting the veil on ultrashort per- and polyfluoroalkyl substances (USC-PFAS) – analysis, occurrence, remediation, and emerging regulations
Ultrashort chain per- and polyfluoroalkyl substances (USC-PFAS), defined as those with three or fewer carbon atoms, are characterised by their significant mobility within the environment, high water solubility, and low affinity for organic matter.

These properties contribute to their widespread presence in environmental waters and wastewaters, as well as drinking waters. The environmental concerns associated with USC-PFAS include their persistence, potential for groundwater contamination, and challenges in remediation due to their resistance to conventional treatment methods.
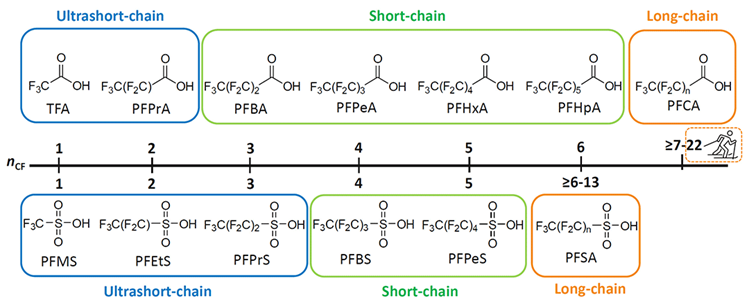
Figure 1: PFSA and PFCA categorization based on carbon chain length – ultrashort-chain, short-chain and long-chain.
(Image provided courtesy of Phenomenex and SCIEX).
Occurrence, uses and sources
Ultrashort chain PFAS have been poorly characterised compared with their longer chain counterparts, and understanding of their intended and unintended transformation processes is limited. Sources of USC-PFAS include degradation of precursor compounds, atmospheric degradation of hydrofluorocarbons and hydrochlorofluorocarbons used as refrigerants, and intentional direct use in products like batteries. They are also byproducts from historical electrochemical fluorination (ECF) manufacture of longer chain PFAS. USCPFAS are also found in urban and industrial waste, as well as aqueous film-forming foams (AFFFs) used in firefighting.

Figure 2: "Conceptual site model for PFASs from a fire training area source zone”, from Managing Risks and Liabilities associated with Per- and Polyfluoroalkyl Substances (PFASs),
CL:AIRE Technical Bulletin, Arcadis 2019. Used with permission.
Fate and transport
A recent survey of USC-PFAS in Australian environmental water samples (Gorji et al.) found ultrashort perfluorocarboxylic acids (USC-PFCAs) including PFPrA in wastewaters, surface waters and groundwaters. Ultrashort perfluorosulfonic acids (USC-PFSAs) were detected in wastewaters, surface waters, groundwaters, and drinking waters (to varying extents and specific to the PFSA compound identified), and hydrogen- substituted PFCAs (USC-H-PFCAs) were identified in surface water.
Carbon chain length plays a key role in determining the physicochemical characteristics of different PFAS chemicals. Long chain PFAS for instance are more hydrophobic, a property that has been leveraged by removal techniques using carbonaceous materials like granular activated carbon (GAC). By contrast, short and ultrashort chain PFAS are hydrophilic, with characteristically low pKa values (i.e. more acidic), high water solubilities and decreased log Koc values (i.e. less adsorptive to organic carbon). These attributes mean that USC-PFAS dissociate freely in aqueous environments and are less prone to sorption onto natural solids. The outcome is that USC-PFAS are highly mobile in the aquatic environment and can travel far from contamination source areas.
The global regulatory landscape
Awareness about the persistence and bioaccumulative nature of C8-C14 PFAS began to attract widespread concern near the turn of the millennium, and inclusion of PFOS, PFOA and PFHxS on the persistent organic pollutants (POPs) list of the Stockholm Convention soon ensued. Consequentially, manufacturers began adopting short and ultrashort chain alternatives.
However, with the change from C8 to C4 and shorter fluorinated carbon chains came the sacrifice to lower technical performance, and greater quantities were needed for equivalent performance. Hence, while it is generally accepted that short and ultrashort chain PFAS have lower toxicological profiles, environmental risks are not necessarily obviated since USCPFAS presence, and continuous, cumulative, release suggests that adverse risks may lie ahead.
Global regulations of USC-PFAS vary by region and are often included under broader PFAS regulations. For example, in the Americas, Europe, and Asia-Pacific (APAC) regions, there are specific guidelines and limits on total PFAS compounds for drinking water, environmental water, and wastewater. In Australia, regulations also cover these areas, with particular attention to the presence of USC-PFAS in aqueous filmforming foams (AFFFs) used in firefighting. Germany has introduced specific regulations for TFA in drinking water, to a level of 60 ug/L, but notes 10 μg/L should be the target threshold, while Denmark’s limit is 9 μg/L, and the Dutch Institute for Public Health (RIVM) set a lower value of 2.2 μg/L.
Targeted analytical services are becoming more accessible
Testing for USC-PFAS compounds is challenging due to several factors. Analytical standards, especially isotopically labelled ones, are hard to find, which complicates the testing process. Background contamination and noise can interfere with the detection of these compounds, especially TFA and other USC-PFCAs. USC-PFSAs and the ether compound PFMOAA have lower ambient background concentrations and lower LOR values are more readily achievable. ALS has achieved a testing method that includes USC-PFAS compounds in environmental water matrices, to the LOR values summarised in table 1 – a more comprehensive compound information sheet is provided as an appendix to this EnviroMail.
| USC-PFAS Compounds and Limits of Reporting (LOR) | CAS No | LOR (μg/L) |
| Difluoroacetic acid (DFA) | 381-73-7 | 1 |
| Trifluoroacetic acid (TFA), Perfluoroethanoic acid (PFEtA) | 76-05-1 | 1 |
| Trifluoromethane sulfonic acid (TFMS), Perfluoromethane sulfonic acid (PFMeS) | 1493-13-6 | 0.01 |
| 2,3,3,3-Tetrafluoropropanoic acid (2333-TFPA) | 359-49-9 | 1 |
| 2,2,3,3-Tetrafluoropropanoic acid (2233-TFPA), Flupropanate | 756-09-2 | 1 |
| Perfluoroethane sulfonic acid (PFEtS) | 354-88-1 | 0.01 |
| Perfluoropropanoic acid (PFPrA) | 422-64-0 | 1 |
| Perfluoro-2-methoxyacetic acid (PFMOAA) | 674-13-5 | 0.1 |
| Perfluoropropane sulfonic acid (PFPrS) | 423-41-6 | 0.01 |
The HDPE sampling containers used for routine PFAS analyte suites are suitable for collecting samples for USC-PFAS. To request analysis for USC-PFAS, please include ALS code EP230 on analysis requisition documents. In the absence of well documented holding time criteria, ALS recommends a conservative holding time of 14-days after sample collection, although stability trials indicate that these analytes can be stable up to several months post sampling.

Get in touch with us
We would love to hear about how our services for this emerging class of PFAS might assist with your project needs, and we welcome feedback on how we might improve or expand our services to meet your needs. Don’t hesitate to get in contact with your nearest ALS laboratory to discuss your PFAS testing requirements.
Brisbane
Sydney
Melbourne
Perth
New Zealand
Download as a PDF