EnviroMail 159 Australia and New Zealand
Fluorotelomer alcohols and related volatile and semi-volatile PFAS compounds – occurrence and pending regulations
Liquid chromatography, coupled with tandem mass spectrometry, in a technique commonly abbreviated to LCMSMS is typically the go to choice for analytical chemists when it comes to PFAS.

Volatile PFAS
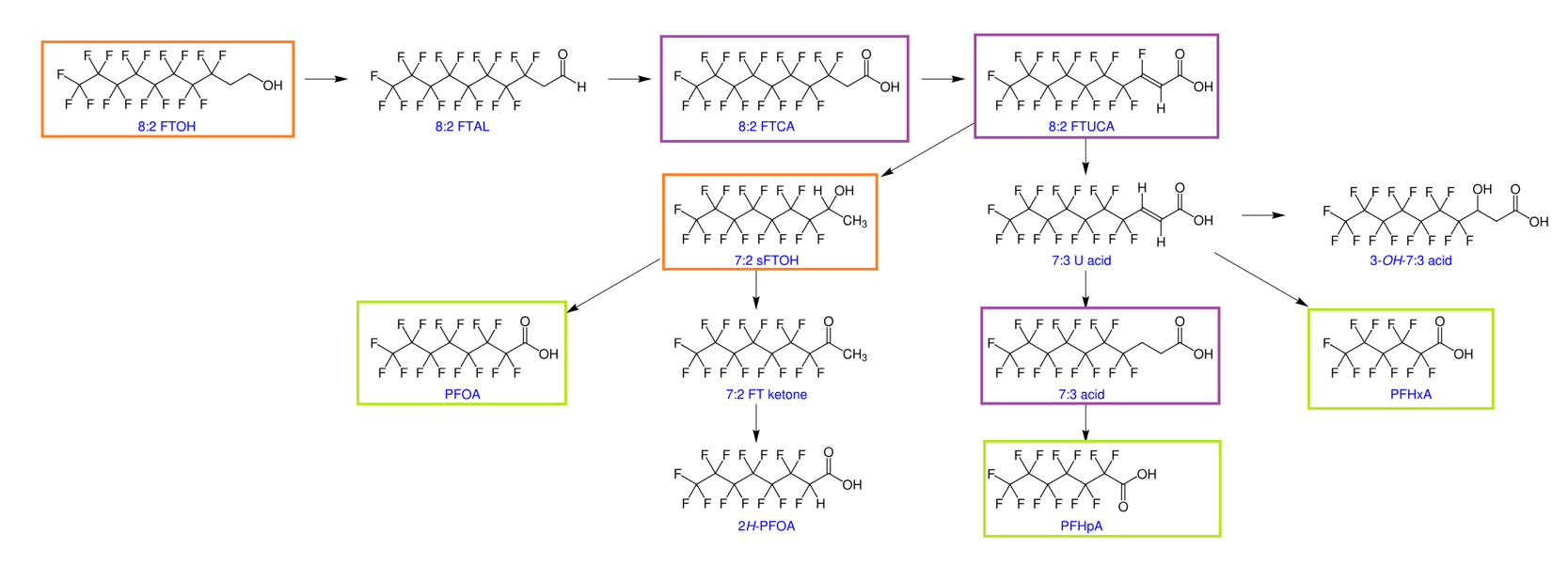
Figure 1: Aerobic biotransformation of 8:2 FTOH in soil – compounds in green boxes are included in ALS’s 30 analyte PFAS suite, compounds in purple boxes are included in ALS’s extended 54 analyte suites, while compounds in orange boxes are included in the new FTOH service offering. 1
1 https://commons.wikimedia.org/wiki/File:Aerobic_biotransformation_8-2_FTOH_soil_labeled.svg.
![]()
Figure 2: SCFPs have been used in resins for semiconductors. A schematic structure of an SCFP and two potential pathways that may liberate 8:2 FTOH and 8:2 FTOAc are displayed here.
Uses and origins
Fluorotelomer alcohols (FTOHs) are polyfluoroalkyl substances and were used heavily in syntheses of surfactants, and as intermediates in the manufacture of diverse products including textiles, polymers, paints, adhesives, waxes, and cleaning agents. They act as surfactants, lubricants and intermediates in manufacturing processes and can be emitted into the atmosphere during production of fluoropolymers.
FTOHs are also a constituent in aqueous film-forming foams (AFFF) and byproducts in fluorotelomer-based AFFFs – 8:2 FTOH concentrations in AFFF were found to range from 8 to 26.5 mg/L (Favreau, 2017). Detection of FTOHs at AFFF-impacted sites is thus likely to increase as analytical methods improve and become more widely available and utilised.
Sidechain fluorinated polymers (SCFPs) comprise another, lesser-known group of PFAS and are characterised by inclusion of fluorinated side chains on larger polymer backbones. The presence of fluorinated side chains helps instil the types of properties to materials for which PFAS have been highly effective, for instance in high temperature resistant, waterproofing and surface coatings formulations.
Fate and transport
Due to the volatility of FTOHs, they can undergo long-range environmental transport. Landfill leachate (Titaley et al., 2023) and wastewater treatment works are potential sources of FTOHs (Wang et al., 2020). FTOHs have been found ubiquitous in water (Ayala-Cabrera et al., 2020; Dimzon et al., 2017), while other studies have shown that FTOHs can breakdown into persistent, bioaccumulative PFCAs by various biotransformation mechanisms (Dinglasan et al., 2004; Ellis et al., 2004; Wang et al., 2009; Yu et al., 2018; Zhao et al., 2013). As such, FTOHs are an indirect source of PFCAs in the environment. In soils, FTOHs have been found to persist for long periods under reducing (redox) conditions. In reducing environments, they may persist for many years (Henderson et al., 2024).
Regulatory interest
European regulations are currently pending for 6:2 and 8:2 FTOH which propose their inclusion within a regulated sum of twenty-four per- and polyfluorinated alkyl substances (PFAS) of primary concern.2 Additionally, the US EPA recently issued a test order for 6:2 fluorotelomer acrylate noting that “even small amounts can significantly contribute to people’s long-term exposure and health risk for cancers, impacts to the liver and heart, and immune and developmental damage to infants and children.”3 Understanding FTOH, FTAC, and FTOAc contamination levels is also important because their degradation products – such as those included in the Stockholm Convention’s list of Persistent Organic Pollutants (POPs) – are regulated widely.

Figure 3: A gull docking model of 8:2 Fluorotelomer acrylate in the binding pocket of transthyretin (a transport protein that transports thyroid hormone and retinol in biochemical processes)4
Testing options for FTOHs, FTACs and FTOAcs
Table 1: ALS Australia now offers the below analyte suite in water and soil samples to the listed LORs.
| Volatile PFAS in water and soil | |||
| Analyte | water LOR (µg/L) | soil LOR (µg/L); | CAS# |
| FBET (4:2 FTOH) | 1 | 20 | 2043-47-2 |
| FHET (6:2 FTOH) | 1 | 20 | 647-42-7 |
| FOET (8:2 FTOH) | 1 | 20 | 678-39-7 |
| FDET (10:2 FTOH) | 1 | 20 | 865-86-1 |
| 5:2 sFTOH (5:2 secondary fluorotelomer alcohol) | 1 | 20 | 914637-05-1 |
| 7:2 sFTOH (7:2 secondary fluorotelomer alcohol) | 1 | 20 | 24015-83-6 |
| 8:2 FTAC | 1 | 20* | 27905-45-9 |
| 10:2 FTAC | 1 | 20* | 17741-60-5 |
| 8:2 FTOAc | 1 | 20* | 37858-04-1 |
| 10:2 FTOAc | 1 | 20* | 37858-05-2 |
*Short holding times apply for these analytes in soil matrices
Sampling and analysis requirements
2 Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Directive 2000/60/EC establishing a framework for Community action in the field of water policy, Directive 2006/118/EC on the protection of groundwater against pollution and deterioration and Directive 2008/105/EC on environmental quality standards in the field of water policy, European Commission, Brussels, Oct 2022.
3 EPA Issues Test Order for PFAS Used in Manufacturing Under National Testing Strategy | US EPA
4 Homology modeling to screen for potential binding of contaminants to thyroid hormone receptor and transthyretin in glaucous gull (Larus hyperboreus) and herring gull (Larus argentatus), (Mortensen et al.).
Get in touch with us
We would love to hear about how our services for this emerging class of PFAS might assist with your project needs, and we welcome feedback on how we might improve or expand our services to meet your needs. Don’t hesitate to get in contact with your nearest ALS laboratory to discuss your PFAS testing requirements.
Brisbane
Sydney
Melbourne
Perth
New Zealand
Download as a PDF