EnviroMail 61 Canada
Isotope Ratio Analysis Solves Complex Problems
The ALS specialty lab in Luleå, Sweden is one of very few laboratories in the world offering commercial isotope ratio testing services not only for radiogenic systems (Sr, Nd, Pu, U) and light stable isotopes (Li, B, Si), but also for heavy stable elements such as Ag, Ca, Cd, Cu, Fe, Mg, Mo, Si, and Zn.

All of these isotope ratio tests can be used as a fingerprint to provide information about origin or geological age, and have potential to be used for tracing pollution and exposure sources.
Introduction to Isotope Ratio Analysis
The isotopic composition of an element can be affected by factors such as its source, exposure to weathering, biological and biochemical processes, or the geological age of a material, and can provide valuable characteristic and diagnostic information.
Isotope ratio analysis is used to accurately measure very small differences in the ratios of different isotopes of an element, and is a powerful tool for many disciplines, such as geology, geochronology, geochemistry, forensic sciences, human nutrition, health studies, and archaeology. Most people are familiar with radiocarbon dating, perhaps the most famous use of isotope ratio analysis, where the ratio of radioactive Carbon-14 to Carbon-12 isotopes can accurately determine the age of organic materials up to around 60,000 years. Isotope Ratio Mass Spectrometry (IRMS) is widely used to measure stable isotope ratios of the most common light elements, including C, N, S, O, and H.
Measurement of stable isotope ratios of heavier elements tends to be more challenging, requiring specialized instrumentation, and typically requiring preconcentration, because heavy element concentrations are very low in most sample types. Some of the most common isotope ratio tests and applications for heavy elements are shown in Table 1. A more extensive overview of heavy element and other non-traditional stable isotope applications for groundwaters can be found in Elemental stable isotope assessment of groundwater contamination: Recent Developments (see References).
Table 1. Common Non-Traditional Isotope Ratio Applications
| Isotope System | Isotopes Measured | Common Isotope Ratio Applications |
| Boron | 10,11B | Enrichment control in the nuclear power industry, tracing pollution sources |
| Lead | 204,206,207,208Pb | Tracing pollution and exposure sources, geology, geochronology, provenance studies, forensics, archaeology |
| Neodymium | 143,144Nd | Geology, geochronology, provenance studies |
| Selenium | 77,78,82Se | Detecting and monitoring post-mining attenuation of Selenium at mining sites |
| Strontium | 86,87Sr | Geology, geochronology, provenance studies, forensics |
| Uranium | 234,235,238U | Enrichment control in the nuclear power industry, tracing pollution and exposure sources |
| Other Heavy Elements | Stable isotopes of Ag, Ca, Cd, Cu, Fe, Mg, Mo, Si, Zn | Geology, tracing pollution and exposure sources |
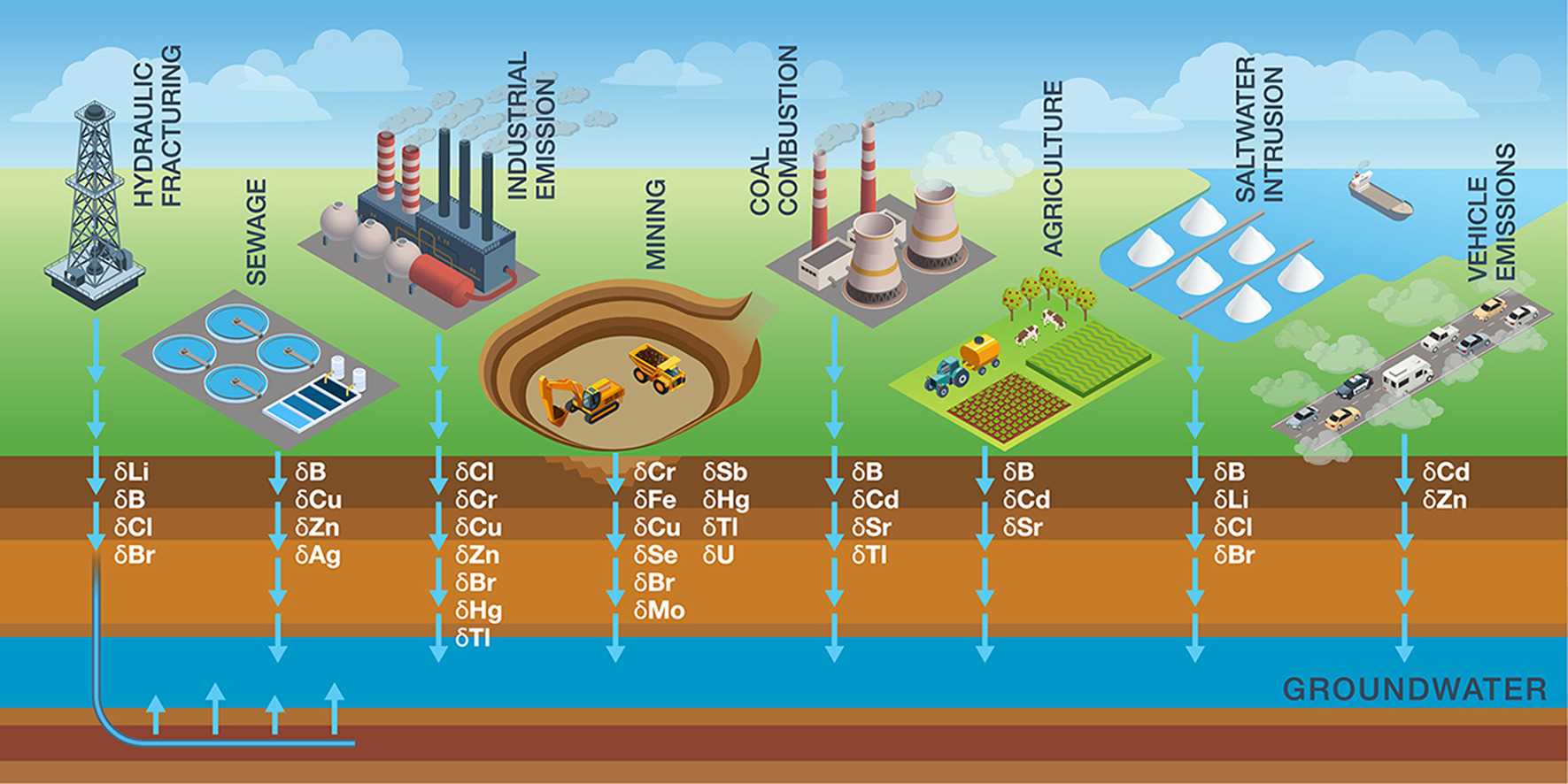
Figure 1: Non-Traditional Stable Isotope Ratio Applications for Groundwater
[Current Opinion in Environmental Science & Health 2022, 26:100330]
ALS Capabilities for Isotope Ratio Analysis
ALS Luleå has more than 30 years of research and commercial testing experience for stable isotope ratio analysis using both High Resolution Sector Field ICP-MS (ICP-SFMS) and Multi-Collector ICP-MS (MC-ICP-MS) instrumentation. Our expert team has made substantial contributions to the field of isotope ratio analysis with 170 peer-reviewed publications. ALS Luleå offers isotope ratio analyses for more than 20 stable and radiogenic isotopic systems in a variety of sample matrices with high precision, even where sample concentrations are very low.
Most isotope ratio tests require high precision, because the differences observed in isotope ratios for most elements are small. MC-ICP-MS is used for the most demanding isotope ratio measurements, where the best possible precision is required. For example, age dating of rocks and meteorites using isotopic ratios of Samarium and Neodymium requires a precision of at least 0.002%, which can only be achieved with MC-ICP-MS. The uncertainties with ICP-SFMS are higher, ranging from about 0.05-1% (depending on test), but are sufficient for some applications. Test results for isotopic ratios are typically reported as delta (δ) values in units of parts per thousand (‰), which relate to internationally accepted reference standards for each isotope system.
Isotope Ratio Testing Requirements and Options
Isotope ratio tests are available for a wide variety of matrices, including (but not limited to) natural and process waters, wastewaters, soils, sediments, aerosols, vegetation, biota, food products, clinical samples, archaeological objects, metals, and alloys.
Isotope ratio tests and application requirements are complex. Experts at ALS Luleå discuss requirements with our clients to determine the most appropriate options, which may require customized sample preparation techniques such as matrix removal, analyte preconcentration, and purification in addition to IRMS analysis. Our chemists can advise about sampling amounts and preparation techniques needed for different matrices to meet the minimum or recommended amounts required for our test methods (as shown in Table 2).
Table 2. ALS Luleå Isotope Ratio Test Options and Required Elemental Amounts

ALS offers the fastest testing available anywhere for isotopic ratio analysis of heavy elements, with routine turnaround times of 6-10 working days (after receipt of samples at ALS Sweden), and with rush analysis possible for some tests – much faster than most university isotope ratio labs.
Please contact your ALS Canada Project Manager for more information about world-class isotope ratio testing options available through ALS Sweden.
References
Elemental stable isotope assessment of groundwater contamination: Recent developments, Ilia Rodushkin, Emma Engström, Simon Pontér, and Maddalena Pennisi, Current Opinion in Environmental Science & Health 2022, 26:100330. https://www.sciencedirect.com/science/article/abs/pii/S2468584422000058
Download this EnviroMail - EnviroMail 61 Canada - Isotope Ratio Analysis Solves Complex Problems