Skin Prick Testing and Exclusive HRIPT Testing Services
Ensuring Consumer Safety and Competitive Advantage in Topical Medical Device Testing
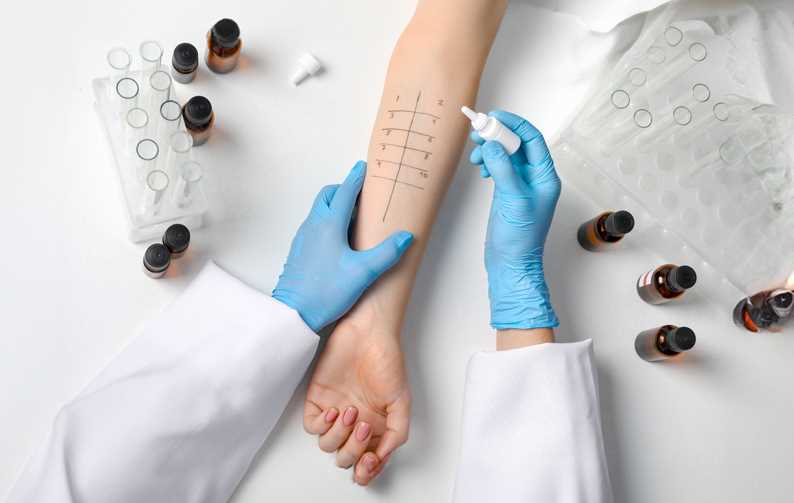
In the competitive world of topical medical devices, ensuring the safety of products is paramount. Not only does it protect consumer health, but it also provides a significant edge for companies that conduct thorough clinical safety tests. Two critical tests in this regard are Skin Prick Testing (SPT) and Human Repeat Insult Patch Testing (HRIPT).
Understanding Skin Prick Testing (SPT)
A Skin Prick Test (SPT), also known as a scratch test, assesses the immunogenic response to a potential allergen. SPT is a vital procedure used to assess the immunogenic response to potential allergens. During this test, a precise amount of the test product is applied to the skin, followed by a prick or scratch. Observations are made at intervals to determine any allergic reactions. This test ensures that the product does not trigger adverse immunogenic reactions, safeguarding consumer health.
How SPT Testing Works?
An allergist and/or nurse applies a precise amount of the test product to the skin, then pricks or scratches the area. This process is repeated with a positive and negative control. Observations of the skin are made at 15 minutes, 6 hours, and 24-48 hours later. The results help determine the allergenic safety of the product, ensuring that the test product and negative control do not trigger immunogenic reactions, while the positive control does.
What is an Exclusive Human Repeat Insult Patch Test (HRIPT)?
The Human Repeat Insult Patch Test (HRIPT) evaluates a formula’s potential to cause irritation, sensitization, and allergenic responses. It's a standard test for personal care products, topically applied medical devices, and both OTC and prescription topical products. The procedure involves repeated application of the product to the participants' backs over three weeks. After a rest period of 7 to 10 days, the product is applied to a new site to check for allergenic responses. The results provide insights into the product’s allergenic safety.
FDA Requirements for Topical Products
To market a medical device in the US, manufacturers must submit a Premarket Notification 510(k) or a Premarket Approval (PMA) application. A 510(k) application must show that the device is substantially equivalent to one already on the market in the United States (predicate device). SPT and HRIPT are essential clinical studies required to demonstrate this equivalence. HRIPT studies should include at least 50 subjects, and SPT should include at least 20. Successful completion of these studies indicates low immunogenic potential of the product.
Why Choose ALS?
Conducting rigorous safety tests like SPT and HRIPT offers a significant competitive advantage for medical device companies. At ALS, quality is paramount, and for our clients, timely report delivery is crucial. We specialize in clinical studies for medical devices and pharmaceutical products to support safety claims and meet regulatory requirements. Here's why ALS stands out:
• Regulatory Compliance: Meeting regulatory requirements is essential for market approval. In the US, for instance, the FDA requires clinical studies to demonstrate the safety of topical products. Successfully completing SPT and HRIPT studies is a key step in this process, ensuring that products meet the necessary safety standards.
• Consumer Trust: Safety testing builds consumer trust. When consumers know that a product has undergone thorough testing, they are more likely to trust and purchase it. This trust translates into brand loyalty and repeat business.
• Market Differentiation: In a crowded market, safety-tested products stand out. Companies that invest in clinical safety testing can differentiate their products from competitors, highlighting their commitment to consumer safety.
• Risk Mitigation: Conducting safety tests helps identify potential issues before products reach the market. This proactive approach reduces the risk of product recalls, legal issues, and damage to the brand’s reputation.
Ensuring the safety of medical device products through rigorous testing like SPT and HRIPT is not just a regulatory requirement but a strategic business decision. It protects consumer health, builds trust, and provides a competitive edge in the market. Companies that prioritize safety testing are well-positioned to succeed in the ever-evolving topical medical device industry.
How ALS Can Help You
ALS is a leader in the safety and efficacy testing of topical medical device products. We work closely with our clients from start to finish to design and deliver custom-tailored studies that meet their needs. Our services include various Safety-in-Use studies, PATCH TESTS (e.g., primary and cumulative irritation), and HRIPT protocols (Modified Draize and Jordan-King procedures).
Our team of expert scientists brings over 200 years of combined experience. ALS has conducted numerous HRIPT and SPT studies for medical device and pharmaceutical clients. Let us help you substantiate the safety of your brand. For inquiries, please email us at pharma.usa@alsglobal.com or call (310) 214-0043.
FDA Links
• https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation
• download (fda.gov)

ALS is expanding PFAS capabilities to include cosmetics testing